A physical property is any measurable property whose value describes the state of a physical system. Changes in a system’s physical properties can be used to describe its transitions between momentary states. The measurement of physical property can alter the arrangement of matter in a sample but not its molecule structure. To put it another way, a physical property may involve a physical change but not a chemical change.
There are two types of physical properties: intensive properties and extensive properties.
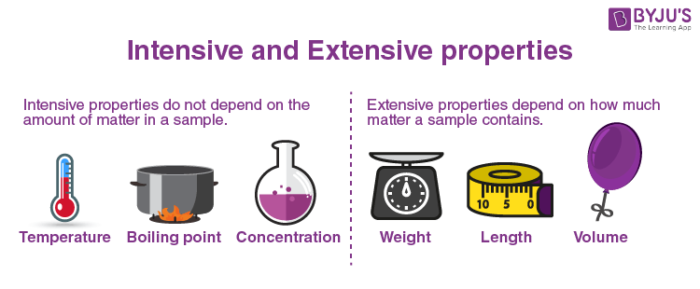
Intensive properties: An intensive property is a bulk property, which means that it is a physical property of a system that is independent of system size or material content. Temperature, refractive index, density, and hardness of an object are examples of intensive properties.
For example- When a diamond is cut, the individual pieces retain their inherent hardness (until their size reaches a few atoms thick).
Extensive properties: For independent, non-interacting subsystems, an extensive property is additive. The property varies with the amount of material in the system.
The examples of extensive properties, such as mass and volume, are affected by the amount of matter measured.
Extensive properties are external, which means they cannot be used to identify the substance, and their value varies depending on the amount of the substance present. You can measure 10g of oil or 10g of water, for example, but this does not allow you to identify a substance as oil or water.

A physical change occurs without any changes in molecular composition. The same element or compound is present both before and after the change. Throughout the changes, the same molecule is present. Because some measurements necessitate changes, physical changes are linked to physical properties.
Some examples of physical changes are-
Some examples of physical properties are:
All physical quantities must be measured. The value of a physical quantity can be expressed as the product of its numerical value and the unit in which it is expressed. The concept of significant figures is used to express the number’s accuracy. S.I. units are used to express measurement units.
Matter refers to everything we see and touch around us. Every matter has unique properties and characteristics that aid in classification and identification.
The primary distinction between Physical and Chemical Properties is that when a chemical reaction occurs on a substance, its molecular structure changes. Whereas a substance’s physical properties can be identified as its chemical composition and identity.